
As the Year of the Horse unfolded, InnoStar continued to stride forward with purpose, leaving meaningful milestones along the vibrant journey of innovative drug development. We are proud to share our 2025 Annual Review—a testament to our commitment to scientific excellence and partnership.
Facility Expansion
This year marked the operational launch of our Nantong Phase II project. With over 20,000 sqm of space and 130 international-standard animal rooms, this facility strengthens our platform to support cutting-edge therapies and global biopharmaceutical growth.

Laboratory Accreditations
Talent growth
Our team now boasts a strong portfolio of professional credentials, with 6 DABT-certified toxicologists, 11 CSQA-accredited GLP-QA professionals, and 21 DCCVP-certified veterinary pathologists on board.
Global Project Success
In 2025, we have achieved the following outstanding milestones (internal data and industry database compilation)

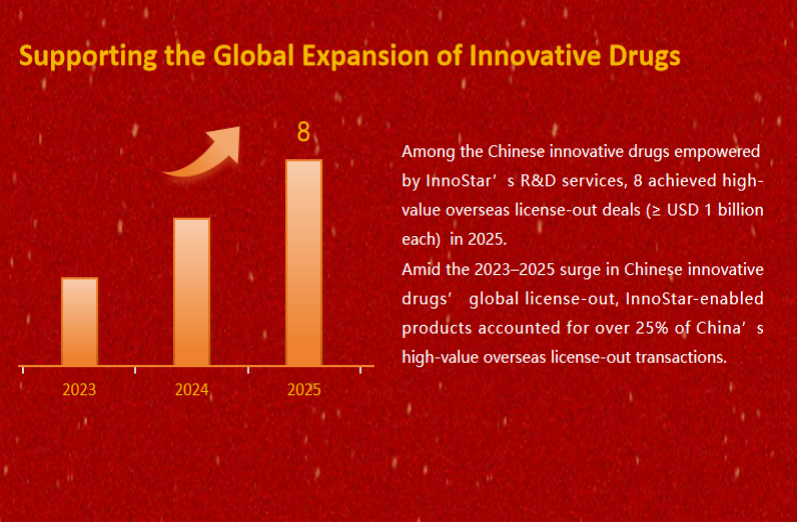
Supporting the Global Expansion of Innovative Drugs
Among the Chinese innovative drugs empowered by InnoStar’s R&D services, 8 achieved high-value overseas license-out deals (≥ USD 1 billion each) in 2025.
Driving First-in-Class Breakthroughs
In 2025, we successfully supported over 10 “first-in-class” breakthroughs globally. Representative pioneering projects are as follows

Global Presence & Industry Engagement
We participated in leading international conferences, sharing insights in immuno-oncology, ADC development, and more. Through events and collaborations, we foster open dialogue and shared growth within the global scientific community.

This annual review captures a year of dedication, achievement, and partnership. We extend our gratitude to every collaborator who trusted us along the way.
Onward to new horizons—InnoStar remains steadfast in paving the way for the next wave of medical innovation.
Copyright © 2017-2021©Shanghai InnoStar Bio-tech Co., Ltd. All Rights Reserved. Created By VPABrand.com